Hearing loss is a common sensory impairment in humans, and there are currently no pharmaceutical treatments available for hereditary hearing loss. Although recent clinical trials of gene therapy for congenital deafness have achieved success, the field still lacks standardized guidelines.

On October 23, 2025, the world's first international expert consensus on gene therapy for hereditary hearing loss was officially published in Med, a medical journal under Cell Press, and was featured as the headline news on the Cell Press website.
This consensus was jointly developed over more than a year, led by the team of Professor Shu Yilai from the Eye & ENT Hospital of Fudan University, in collaboration with Professor Zheng-Yi Chen from Harvard Medical School, Professor Chai Renjie from the School of Life Science and Technology at Southeast University, Professor Lawrence R. Lustig from Columbia University Medical Center, and 46 multidisciplinary experts from countries including China, Spain, the UK, USA, South Korea, and Germany, spanning fields such as otology, genetics, audiology, gene therapy, and auditory rehabilitation.

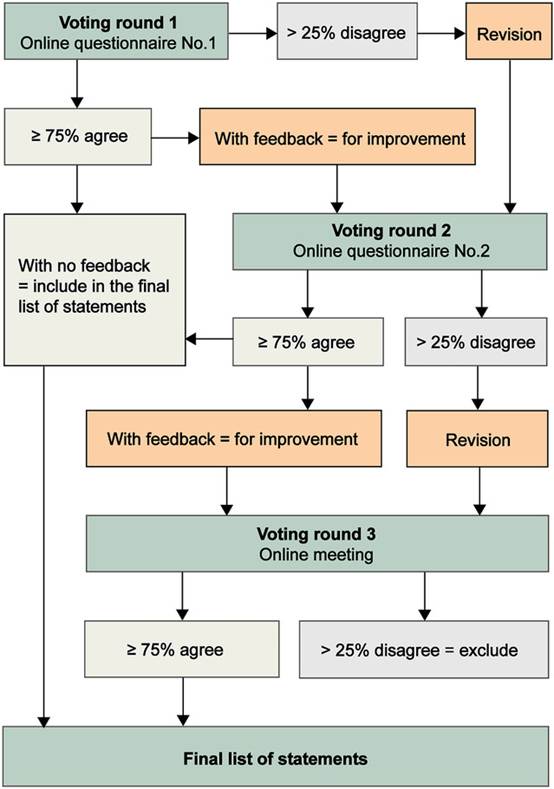
Strictly based on a modified Delphi method, the consensus process established an expert steering committee comprising Professor Fan-Gang Zeng from the University of California, Irvine (Member of the US National Academy of Engineering), Professor Lukas D. Landegger from Stanford University School of Medicine, Professor Tobias Moser from the University Medical Center Göttingen, Editor Jin Xin from the Chinese Journal of Otorhinolaryngology Head and Neck Surgery, and Professor Sun Yu from Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. Under the guidance and review of this committee, and through two rounds of questionnaire surveys and one consensus meeting held between March 2024 and March 2025, 30 consensus statements were finalized. These statements cover six key modules: ethical review, patient selection, preoperative diagnosis and assessment, gene therapy drug delivery, postoperative follow-up, and auditory-verbal rehabilitation, providing the first standardized reference framework for the clinical application of gene therapy for hereditary hearing loss worldwide.
According to WHO (2025) statistics, over 430 million people globally suffer from disabling (moderate or greater) hearing loss, including approximately 34 million children. Genetic factors account for up to 60% of congenital hearing loss cases.
In recent years, gene therapy clinical trials targeting the deafness gene OTOF are actively advancing in several countries, including China, the USA, Spain, the UK, and France, and have effectively restored hearing and speech function in subjects in early-stage clinical trials.
As the lead of the world's first team to initiate a gene therapy clinical trial for congenital deafness, and the initiator and senior corresponding author of this consensus, Professor Shu Yilai from the Eye & ENT Hospital of Fudan University stated: "Gene therapy brings revolutionary hope for hereditary hearing loss, especially for DFNB9 deafness caused by OTOF gene mutations, which has been launched in multiple countries and shown efficacy. However, unified clinical implementation standards have not yet been established globally. The experience gained in this field impels us to take the responsibility of uniting global experts and scholars committed to related research to establish a consensus on gene therapy for hereditary hearing loss, promoting the standardization and normalization of clinical trials and applications."
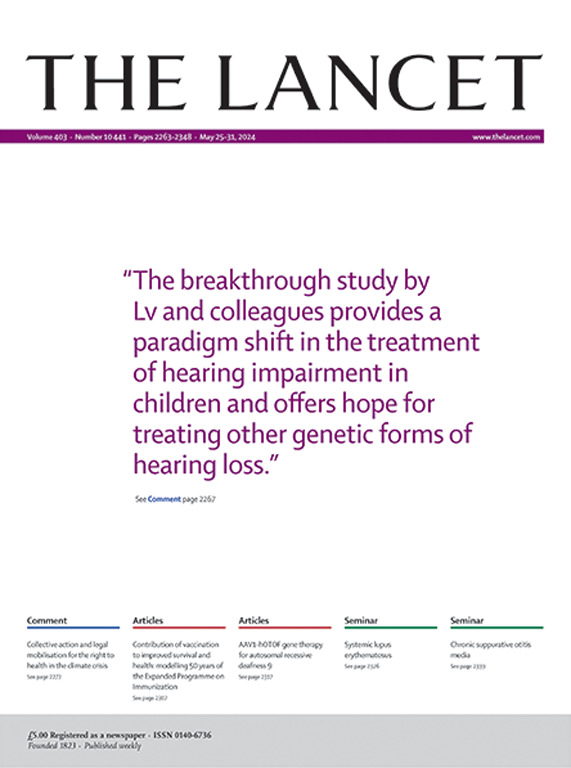
This team previously developed an OTOF deafness gene therapy and initiated the world's first gene therapy clinical trial for congenital deafness in 2022. It has successfully restored hearing, speech, and sound localization abilities in dozens of OTOF deafness patients. Related results have been published in top-tier medical journals such as The Lancet, Nature Medicine, JAMA Neurology, Nature Human Behaviour, and Med, and were selected for the cover highlight of The Lancet. They were evaluated by international peers as "a paradigm shift in treating children with hearing impairment and bringing hope for treating other genetic forms of hearing loss," "marking the dawn of a new era for gene therapy for hearing impairment and more broadly," and "a landmark breakthrough in the auditory field."
The consensus clearly states that patient selection should be based on a clear genetic diagnosis, with pathogenic or likely pathogenic variants requiring independent diagnosis by at least two clinical geneticists; priority should be given to patients with severe, profound, or complete hearing loss. Furthermore, the consensus provides specific guidance on surgical approaches (via transmastoid facial recess or external auditory canal), perioperative medication (such as the use of corticosteroids and antibiotics), long-term follow-up (recommended for at least 5 years), and auditory-verbal rehabilitation, offering important references for clinical practice.
Professor Lawrence R. Lustig from Columbia University emphasized: "This is an important milestone in the field of deafness gene therapy. For the first time, we have constructed a globally unified guiding framework for deafness gene therapy clinical trials, providing a solid guarantee for the steady development of this field based on safety, science, and collaboration."
Professor Zheng-Yi Chen from Harvard Medical School pointed out: "Deafness gene therapy has the potential to transform the treatment landscape for hereditary hearing loss. As the field advances rapidly, the release of this consensus provides crucial guidance for the safe and effective conduct of future clinical trials."
Professor Chai Renjie from the School of Life Science and Technology at Southeast University added: "A mature guiding framework for gene therapy in the field of hearing loss was previously lacking. The release of this consensus not only fills this gap but also provides an important reference for gene therapy of other rare diseases."
This consensus provides the first globally recognized framework for gene therapy of hereditary hearing loss, comprising 30 consensus statements covering six key modules of hereditary hearing loss gene therapy: Ethical Review (1 statement), Patient Selection (12 statements), Preoperative Diagnosis and Assessment (9 statements), Gene Therapy Drug Delivery (4 statements), Postoperative Follow-up (3 statements), and Post-treatment Auditory and Speech Rehabilitation (1 statement).
The consensus standardizes the design of clinical trials and patient management for hereditary hearing loss gene therapy, accelerates the translation from research to practice, while ensuring safety. This consensus can be immediately applied to OTOF-related hearing loss and can be adapted for other genetic forms.
The consensus is expected to serve as an important reference for clinicians, researchers, pharmaceutical companies, ethics committees, and regulatory agencies. As more clinical trials are conducted and related data accumulates, the consensus content will be continuously updated and refined to provide more comprehensive guidance for the development of this field.
Professors Shu Yilai (Eye & ENT Hospital of Fudan University), Zheng-Yi Chen (Harvard Medical School), Chai Renjie (Southeast University), and Lawrence R. Lustig (Columbia University Medical Center) are the co-senior corresponding authors of the consensus. Fan Xintai, Gao Ziwen, Zhong Jiake, Chen Yuxin, Chen Xiaoyun (all from the Eye & ENT Hospital of Fudan University), Professor Lukas D. Landegger (Stanford University School of Medicine), and Professor Tobias Moser (University Medical Center Göttingen) are the co-first authors of the paper. The research received funding from multiple sources, including the National Natural Science Foundation of China and the German Research Foundation (DFG). (Adapted from the "Biodiscover" WeChat official account)
Full text link: https://www.cell.com/med/fulltext/S2666-6340(25)00313-7
