On June 18, 2025, the team led by Principal Investigator Lin Chengqi from the School of Life Science and Technology at Southeast University made a significant breakthrough in the field of mammalian organ development. The related findings were published in the prestigious international academic journal Cell under the title, "Digital Reconstruction of Full Embryos During Early Mouse Organogenesis."
In mammalian embryonic development, the three germ layers—endoderm, mesoderm, and ectoderm—derived from gastrulation drive organogenesis through synergistic interactions. In 1924, through amphibian embryo transplantation experiments, Spemann and Mangold discovered the "organization centre" that regulates the formation of ectodermal organ primordia and the body axis, for which they were awarded the Nobel Prize in Physiology or Medicine in 1935. However, despite breakthroughs in the study of the organization center for ectodermal organs, the precise localization of primordia for mesodermal and endodermal organs, including the heart, and the study of their microenvironments have long been challenging.
Supported by the National Key R&D Program of China and the National Natural Science Foundation of China, the team of Lin Chengqi, Luo Zhuojuan, and Xie Peng at Southeast University, in collaboration with several domestic institutions, has achieved a major breakthrough in the construction of single-cell digital embryos. They have pioneered the world's first three-dimensional digital embryo with single-cell precision, discovered the Primordium Determination Zone (PDZ) for mesodermal and endodermal organs, and successfully deciphered the code of heart development (Figure 1). This discovery not only elucidates the unique signaling network in the microenvironment that governs the formation of mesodermal and endodermal organ primordia but also fills a theoretical gap in our understanding of the early developmental mechanisms of the mammalian heart. It provides a critical theoretical foundation for the prevention and treatment of congenital birth defects such as congenital heart disease, as well as for regenerative medicine.
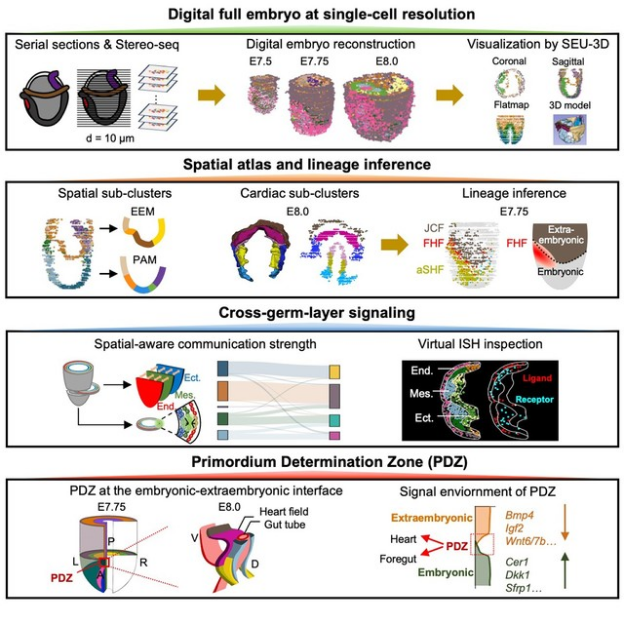
Figure 1. Deciphering the code of early heart development using single-cell digital embryo technology.
To address the core question of how organ primordia originate from the germ layers, the research team constructed 3D digital embryos with single-cell resolution, covering the period from late gastrulation to the formation of the heart and other organ primordia in mice. This allowed them to analyze the dynamic developmental atlas of early mouse cardiac and other mesodermal and endodermal organs. Based on this, they focused on the dynamics of the cellular microenvironment and discovered a unique signaling "low-land" at the embryonic-extraembryonic boundary at embryonic day 7.75 (E7.75) in mice—a critical window for the formation of mesodermal and endodermal organ primordia. They named this region the Primordium Determination Zone (PDZ). The adjacent embryonic and extraembryonic tissues to the PDZ exhibit high concentrations of signaling inhibitory molecules and activating ligands, respectively. However, the PDZ itself presents a "low-land" of low signaling activity while expressing a variety of receptor signaling genes. This creates a microenvironment receptive to regulatory inputs from multiple germ layers, driving the coordinated development of the heart and foregut primordia. Through spatial transcriptomics, this study reveals for the first time the central role of the PDZ as a hub that converges signals across germ layers. By integrating WNT, BMP, and FGF pathways, the PDZ translates microenvironmental signals into instructions for selective gene expression, thereby driving the formation of organ primordia. This finding not only clarifies the unique microenvironmental signaling network for heart primordium formation but also fills the theoretical void in the early developmental mechanisms of the mammalian heart, providing a crucial theoretical basis for the prevention of congenital heart disease and for regenerative medicine.
Dr. Xie Peng, a Research Professor at the School of Biological Science and Medical Engineering at Southeast University; Dr. Shen Juan, an Associate Research Scientist at BGI-Research; Dr. Yang Yi, an Associate Professor at the School of Life Science and Technology at Southeast University; Dr. Wang Xinrui, a Research Professor at Fujian Maternity and Child Health Hospital; Dr. Liu Wei from The Chinese University of Hong Kong, Shenzhen; and Dr. Cao Hailong, a Professor at Zhongda Hospital, Southeast University, are the co-first authors of the paper. Professor Lin Chengqi from the School of Life Science and Technology at Southeast University; Dr. Fang Xiaodong, a Research Scientist at BGI-Research; Professor Luo Zhuojuan from the School of Life Science and Technology at Southeast University; Professor Cao Hua from Fujian Maternity and Child Health Hospital; and Professor Liu Jin from The Chinese University of Hong Kong, Shenzhen, are the co-corresponding authors. Professor Xie Wei from the School of Life Science and Technology at Southeast University; Professor Yao Xuebiao and Professor Liu Xing from the School of Life Sciences at the University of Science and Technology of China; and Professor Jing Naihe and Associate Research ProfessorYang Xianfa from Guangzhou Laboratory also participated in this research. This work was funded by the National Key R&D Program of China and the National Natural Science Foundation of China.
(Contribution from: The Lin Chengqi and Luo Zhuojuan Team; Reviewed by: Chen Lei)
