Recently, Professor Ling Lin's team from the School of Life Science and Technology atSoutheast University published a research paper online in the journal Cell Reports titled, "miRNA-GABA influences female Aedes aegypti reproduction by modulating midgut homeostasis." Using a comprehensive suite of experimental methods including digestive physiology assays, small RNA sequencing, CRISPR-Cas9 gene editing, RNA interference, and in vitro amino acid delivery, the study demonstrates that the miR-7-GAD-GABA signaling axis mediates the reproductive cascade of the midgut-fat body-ovary axis by regulating midgut homeostasis.
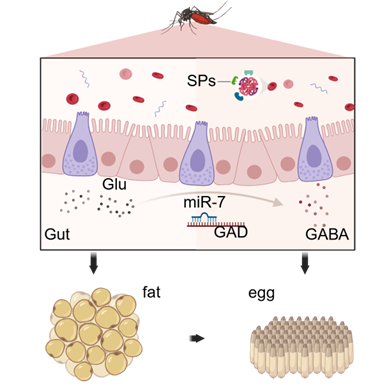
The powerful reproductive plasticity of female Aedes aegypti mosquitoes after a blood meal exacerbates the transmission of mosquito-borne viruses, placing a heavy burden on global public health. A blood meal activates synergistic changes along the midgut-fat body-ovary axis, building up nutritional reserves for the female mosquito's reproductive behavior. MicroRNAs (miRNAs) precisely control gene expression through post-transcriptional regulatory mechanisms that target messenger RNAs (mRNAs).
This study reveals an important miRNA-mediated mechanism. The research demonstrates that miR-7 regulates the level of GABA in the midgut, thereby maintaining intestinal homeostasis. Small RNA sequencing identified miR-7 as a key regulatorymolecule sensitive to the dynamics of blood meal digestion. Mechanistically, miR-7 directly targets glutamate decarboxylase (GAD) to regulate the metabolic balance of glutamate-GABA in the midgut. The study also found that disrupting this mechanism in female mosquitoes leads to impaired protease activity in the midgut, disordered lipid storage in the fat body, and reduced fecundity. This research delineates a hierarchical regulatory framework where the miR-7-GABA axis modulates the efficiency of blood meal digestion and ultimately determines reproductive fitness. The study identifies a crucial miRNA that affects reproductive output by regulating digestion, providing a key target for interventions aimed at blocking vector-borne virus transmission.
Professor Ling Lin from the School of Life Science and Technology at Southeast University is the corresponding author of this paper, and doctoral student Cao Jianping is the first author. This research was supported by the National Natural Science Foundation of China. (Contributed by Cao Jianping)
Paper Link:https://www.cell.com/cell-reports/fulltext/S2211-1247(25)00547-9
5.Professor Han Junhai’s Team Publishes New Findings in Neuroscience Bulletin
Posted by: Wu Zhilong | Date: May 7, 2025 | Views: 334
On April 30, 2025, the research team led by Professor Han Junhai from the School of Life Science and Technology, Southeast University, and the Key Laboratory of “Genes Related to Development and Disease” of the Ministry of Education, published a research article titled"The Glutamate-gated Chloride Channel Facilitates Sleep by Enhancing the Excitability of Two Pairs of Neurons in the Ventral Nerve Cord of Drosophila" in the journalNeuroscience Bulletin. The study elucidates the neural mechanisms by which the glutamate-gated chloride channel (GluClα) facilitates nocturnal sleep.
Sleep is an essential physiological process for human survival. Sleep disorders can result in metabolic imbalance, compromised immunity, emotional disturbances, and, in severe cases, death. The precise regulation of neurotransmitter systems is critical for maintaining normal sleep. Glutamate, the brain's principal excitatory neurotransmitter, has been implicated in both sleep and wakefulness regulation; however, its exact role remains unclear. UsingDrosophila melanogaster as a model, this study employed a combination of genetic manipulation, immunohistochemistry, and sleep behavior analysis to demonstrate for the first time that two specific pairs of neurons in the ventral nerve cord (VNC) promote nocturnal sleep by expressing GluClα to receive inhibitory glutamatergic input from the brain. This work reveals the underlying neural circuitry and molecular mechanisms of this sleep-promoting pathway. The findings not only confirm that glutamate can participate in sleep regulation by transmitting inhibitory signals via GluClα, thereby expanding the conventional understanding of glutamatergic neurotransmission, but also highlight the involvement of the peripheral nervous system in the sleep-regulating network. These insights provide a new perspective on the neurobiology of sleep.
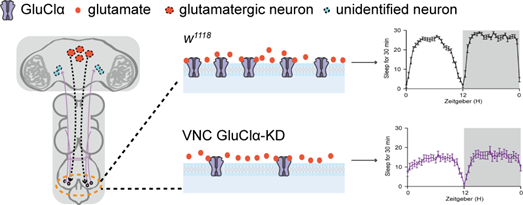
Figure 1. Mechanism of sleep regulation by the glutamate-gated chloride channel. Under normal conditions, two pairs of VNC neurons receive glutamatergic input, which inhibits their activity via GluClα and promotes nocturnal sleep. Loss of GluClα reduces this inhibitory input, rendering the neurons more excitable and leading to decreased sleep in flies.
Associate Research Professor Tian Yao and Professor Han Junhai are the corresponding authors of this study, with doctoral student Fan Yaqian as the first author. This research was supported by the Major Project of Science and Technology Innovation 2030 – “Brain Science and Brain-Inspired Research” and the National Natural Science Foundation of China. (Source: School of Life Science and Technology)
Paper link:https://link.springer.com/article/10.1007/s12264-025-01397-1
