On July 2, 2025, Professor Chai Renjie from the School of Life Science and Technology at Southeast University, in collaboration with several institutions, published a research paper in the top-tier international medical journalNature Medicine titled, "AAV gene therapy for autosomal recessive deafness 9: a single-arm trial." The study reports that AAV gene therapy demonstrates good long-term safety and efficacy in a cohort of DFNB9 patients spanning ages 1.5 to 23.9 years. It also reveals that the age of 5-8 years may be the optimal intervention window, a finding of significant clinical guidance value.

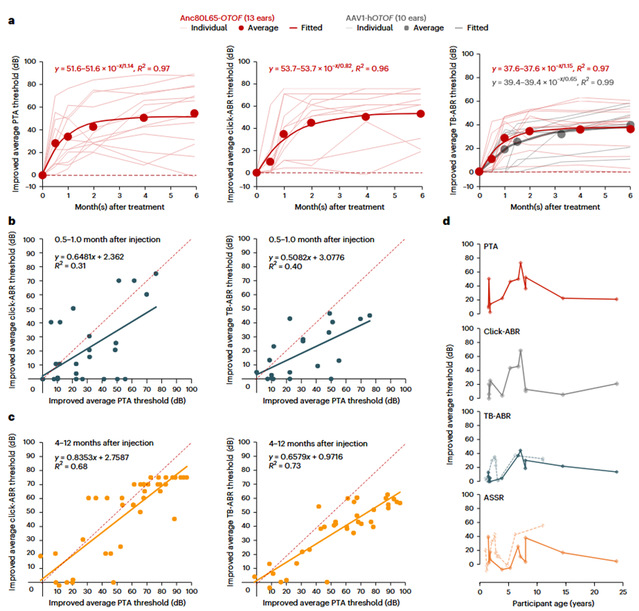
Figure: Changes in hearing of subjects after gene therapy.
Mutations in theOTOF gene are the primary cause of autosomal recessive deafness type 9 (DFNB9), where patients lose their hearing due to defective synaptic vesicle function in inner ear hair cells. Cochlear implants are the main treatment for patients with profound DFNB9 deafness. Although they can partially restore hearing, they have limitations such as distorted sound quality and difficulty in sound recognition in noisy environments.OTOF gene therapy, however, targets the root cause of the disease by precisely delivering the functionalOTOF gene to the inner hair cells of the cochlea via an adeno-associated virus (AAV) vector, thereby restoring synaptic transmission. Previously, Professor Chai Renjie's team at Southeast University had already demonstrated its safety and efficacy in non-human primates and young children, with subjects achieving near-normal hearing in the best cases (Advanced Science 2023, 2024;The Lancet 2024). However, key scientific questions remained unresolved: Is this therapy applicable to older patients? Is there an optimal therapeutic age window?
To answer these questions, Professor Chai Renjie's team conducted a further multi-center clinical trial in China, including Shandong Provincial Second People's Hospital (Shandong Provincial ENT Hospital), Nanjing Drum Tower Hospital (the Affiliated Hospital of Nanjing University Medical School), Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Xijing Hospital of the Fourth Military Medical University, and Ningbo No. 2 Hospital. For the first time, the trial included adolescent and adult subjects, recruiting a total of 10 patients withOTOF-related deafness aged 1.5-23.9 years. The study lasted for 12 months, with safety closely monitored through ear examinations and blood tests, while hearing improvement was measured using objective tests (Click and tone-burst ABR, Auditory Steady-State Response [ASSR]) and behavioral assessments (Pure-Tone Audiometry [PTA]). During the 12-month follow-up period, the 10 patients reported a total of 162 grade I/II adverse events, with no grade III or higher adverse reactions, strongly confirming the safety of the gene therapy. The average pure-tone hearing threshold for all subjects significantly decreased from a pre-operative level of 106 dB to 52 dB (the level of normal conversation), with 62% of this improvement occurring within the first month of treatment. Within 6 months post-operation, some pediatric subjects achieved normal auditory function (<20 dB), while adolescent and adult patients saw their average pure-tone thresholds improve by 46 dB and 36 dB, respectively. Two of the subjects achieved near-normal speech perception. These results indicate that although auditory pathway plasticity decreases with age, gene therapy still has significant efficacy in adolescents and adults. Furthermore, the study found that the auditory brainstem response at 4 months post-treatment can serve as an effective objective predictor of behavioral hearing, indicating that synaptic repair has been completed. Another important finding of this study is that children aged 5 to 8 benefit the most. Compared to toddlers (1-5 years), adolescents, and adults, patients in this age group showed better hearing improvement across multiple audiological indicators. This counter-intuitive clinical finding was surprising—theoretically, better-preserved inner ear integrity and function at an earlier age should predict a better treatment response. However, these findings suggest that the brain's ability to process newly restored sound may vary with age, for reasons that are yet to be explored.
In summary, the important findings of this study not only extend the therapeutic window for DFNB9 gene therapy but also identify the optimal age range for treatment, which can be used to guide clinical intervention timing and strategy development.
The co-corresponding authors of the paper are Professor Chai Renjie from the School of Life Science and Technology at Southeast University; Professor Zeng Fang-Gang from the University of California, Irvine, and a member of the U.S. National Academy of Engineering; Professor Xu Lei from Shandong Provincial Second People's Hospital (Shandong Provincial ENT Hospital); Professor Gao Xia from Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School; Professor Sun Yu from Union Hospital, Tongji Medical College, Huazhong University of Science and Technology; Professor Zha Dingjun from Xijing Hospital, Fourth Military Medical University; and Professor Maoli Duan from Karolinska University Hospital, Sweden. The co-first authors are Associate Professor Qi Jieyu from Beijing Institute of Technology; Dr. Zhang Liyan, a postdoctoral fellow at Zhongda Hospital, Southeast University; Professor Lu Ling; Associate Research Professor Tan Fangzhi; doctoral student Lu Yicheng; and Assistant Research Professor Cheng Cheng from Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School. (Source: School of Life Science and Technology)
Paper Link:https://www.nature.com/articles/s41591-025-03773-w
